
What is the balanced chemical equation for the reaction used to calculate ΔH∘fΔHf∘ of BaCO3(s)BaCO3(s)? If fractional coefficients are required, enter them as a fraction (i. e. 1/3). Indicate the physical states using the abbreviation (ss), (ll), or (gg) for solid, liquid, or gas, respectively without indicating allotropes. Use (aqaq) for aqueous solution.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lilyclairehutson
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 10:50, mi364
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3


Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1
You know the right answer?
What is the balanced chemical equation for the reaction used to calculate ΔH∘fΔHf∘ of BaCO3(s)BaCO3(...
Questions in other subjects:


Social Studies, 22.08.2019 08:30

History, 22.08.2019 08:30

Chemistry, 22.08.2019 08:30


History, 22.08.2019 08:30




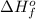 :
: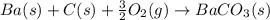
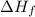 ) is defined as change in enthalpy associated with formation of 1 mole of compound from its constituting elements present in their standard state.
) is defined as change in enthalpy associated with formation of 1 mole of compound from its constituting elements present in their standard state.

