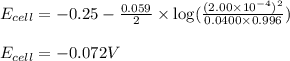Calculate the theoretical potential of the following cell. Indicate whether the reaction will proceed spontaneously in the direction considered (oxidation on the left, reduction on the right) or whether an external voltage source is needed to force this reaction to occur.
Pt, H2(757 torr)|HCl(2.00×10-4 M) parallel to Ni2+(0.0400 M)|Ni
the answer is
-0.072 V; External voltage needed
can anyone explain to me how i get this answer ?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, thatonestudent2271
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1


Chemistry, 22.06.2019 10:00, JOEFRESH10
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 13:00, torigirl4126
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3
You know the right answer?
Calculate the theoretical potential of the following cell. Indicate whether the reaction will procee...
Questions in other subjects:

Mathematics, 03.01.2020 09:31

Mathematics, 03.01.2020 09:31


Mathematics, 03.01.2020 09:31






English, 03.01.2020 09:31

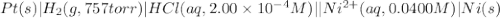
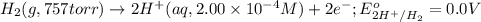
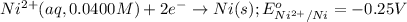
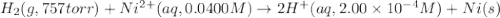
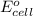 of the reaction, we use the equation:
of the reaction, we use the equation: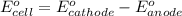
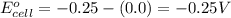
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[H^{+}]^2}{[Ni^{2+}]\times p_{H_2}}](/tpl/images/0549/9866/bc143.png)
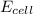 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V![[H^{+}]=2.00\times 10^{-4}M](/tpl/images/0549/9866/69105.png)
![[Ni^{2+}]=0.0400M](/tpl/images/0549/9866/57673.png)
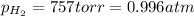 (Conversion factor: 1 atm = 760 torr)
(Conversion factor: 1 atm = 760 torr)