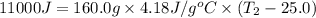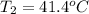Consider the dissolution of CaCl₂: CaCl₂(s) → Ca²⁺(aq) + 2Cl⁻(aq) ∆H = -81.5 kJ/mol A 15.0-g sample of CaCl₂ is dissolved in 145 g water with both substances at 25.0°C. Calculate the final temperature of the solution assuming no heat loss to the surroundings and assuming the solution has a specific heat capacity of 4.18 J/°C・g.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, madlenserlipepu1o
__ _ _ _ _ is the process of removing earth materials from their original sites through weathering and transport and depositing the in another location. a. erosion b. sedimentation c. lithification d. dissolution
Answers: 1

Chemistry, 22.06.2019 11:30, elijah1090
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2
You know the right answer?
Consider the dissolution of CaCl₂: CaCl₂(s) → Ca²⁺(aq) + 2Cl⁻(aq) ∆H = -81.5 kJ/mol A 15.0-g sample...
Questions in other subjects:


Mathematics, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

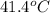
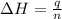
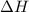 = enthalpy change = 81.5 kJ/mol
= enthalpy change = 81.5 kJ/mol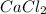 = 15.0 g
= 15.0 g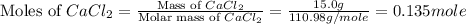
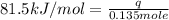
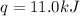
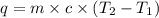
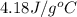
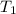 = initial temperature =
= initial temperature = 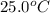
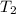 = final temperature = ?
= final temperature = ?