
Chemistry, 17.03.2020 01:09 haileeattaway
The reaction between NO2 and CO to produce NO and CO2 is believed to occur via two steps: step 1: NO2 + NO2 → NO + NO3 step 2: NO3 + CO → NO2 + CO2 The experimental rate law is rate = k[NO2]2 (a) Write the equation for the overall reaction.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, angelrenee2000
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2


Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1
You know the right answer?
The reaction between NO2 and CO to produce NO and CO2 is believed to occur via two steps: step 1: NO...
Questions in other subjects:

Mathematics, 12.10.2019 07:30



History, 12.10.2019 07:30






History, 12.10.2019 07:30

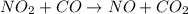
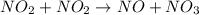
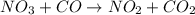
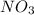 is produced in the first step and subsequently consumed in second step. Hence,
is produced in the first step and subsequently consumed in second step. Hence, 

