
Chemistry, 17.03.2020 00:57 claytonhopkins
A 1.50 L buffer solution is 0.250 M in HF and 0.250 M in NaF. Calculate the pH of the solution after the addition of 0.100 moles of solid NaOH. Assume no volume change upon the addition of base. The Ka for HF is 6.8 × 10-4.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, hdjsjfjruejchhehd
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1


Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 18:00, liddopiink1
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
A 1.50 L buffer solution is 0.250 M in HF and 0.250 M in NaF. Calculate the pH of the solution after...
Questions in other subjects:




Mathematics, 20.03.2021 01:10



Mathematics, 20.03.2021 01:10

Mathematics, 20.03.2021 01:10


Computers and Technology, 20.03.2021 01:10

 .
.
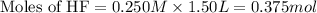
 .
.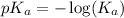
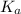 in this expression, we get:
in this expression, we get:



![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0549/7799/e961a.png)
![pH=pK_a+\log \frac{[F^-]}{[HF]}](/tpl/images/0549/7799/bef2f.png)
![pH=3.17+\log [\frac{(\frac{0.475}{1.50})}{(\frac{0.275}{1.50})}]](/tpl/images/0549/7799/a4ae7.png)



