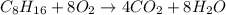Chemistry, 17.03.2020 00:32 moneydee123
Write the balanced equation for the combustion of isooctane (C8H18) to produce carbon dioxide and water. Use the smallest possible integers to balance the equation. Also, separate the sign with 1 space and enter the reaction arrow as hyphen greater than sign: ->

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, noathequeen
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 22:30, wpatskiteh7203
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

You know the right answer?
Write the balanced equation for the combustion of isooctane (C8H18) to produce carbon dioxide and wa...
Questions in other subjects:

Health, 19.07.2019 22:30


Mathematics, 19.07.2019 22:30







Social Studies, 19.07.2019 22:30