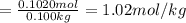Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, officialgraciela67
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1



You know the right answer?
A solution of phosphoric acid was made by dissolving 10.0 g of H3PO4 in 100.0 mL of water. The resul...
Questions in other subjects:

Mathematics, 08.02.2021 22:40

Mathematics, 08.02.2021 22:40

History, 08.02.2021 22:40



Chemistry, 08.02.2021 22:40

Biology, 08.02.2021 22:40


Mathematics, 08.02.2021 22:40

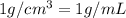
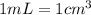
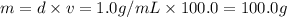
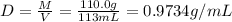
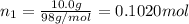
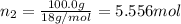
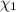
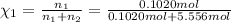

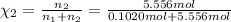
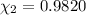
![[Molarity]=\frac{\text{Moles of solute}}{\text{Volume of solution(L)}}](/tpl/images/0549/6266/0dac6.png)

![[Molality]=\frac{\text{Moles of solute}}{\text{Mass of solvent(kg)}}](/tpl/images/0549/6266/71fd2.png)