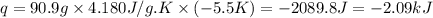Chemistry, 16.03.2020 22:55 brendacauani12345
F 65mL of sulfuric acid and 25mL of sodium hydroxide were mixed and the solution had a density of 1.01g/mL, what is the heat of the calorimeter in kJ given the temperature change of the above equation. You may assume the solution has a heat capacity of 4.180J/gK. Express your final answer in kJ and with 2 decimal places

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 13:30, princessroseee769
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
F 65mL of sulfuric acid and 25mL of sodium hydroxide were mixed and the solution had a density of 1....
Questions in other subjects:

Mathematics, 28.05.2020 02:07

Mathematics, 28.05.2020 02:07

English, 28.05.2020 02:07


Mathematics, 28.05.2020 02:07

History, 28.05.2020 02:07


English, 28.05.2020 02:07


Mathematics, 28.05.2020 02:07

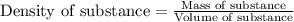
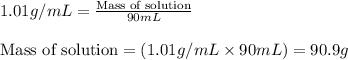
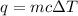
 = change in temperature = -5.5 K
= change in temperature = -5.5 K