
Chemistry, 16.03.2020 22:21 lerasteidl
Calculate the pH of each of the following solutions. a. 0.100 M propanoic acid (HC3H5O2, Ka 1.3 105 ) b. 0.100 M sodium propanoate (NaC3H5O2) c. pure H2O d. a mixture containing 0.100 M HC3H5O2 and 0.100 M NaC3H5O2

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, janayflowers042
Order the following from smallest to largest atom, electron, quark, proton, neutron, molecule, nucleus
Answers: 1


Chemistry, 22.06.2019 11:00, 21villalobosjabez
Which type of fossil does this image depict?
Answers: 1

You know the right answer?
Calculate the pH of each of the following solutions. a. 0.100 M propanoic acid (HC3H5O2, Ka 1.3 105...
Questions in other subjects:


Mathematics, 03.02.2020 00:56

Mathematics, 03.02.2020 00:56

Biology, 03.02.2020 00:56






![K_{a} = \frac{[C_{3}H_{5}O_{2}^{-}][H_{3}O^{+}]}{[C_{3}H_{6}O_{2}]}](/tpl/images/0549/3755/fa55a.png)
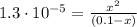 (2)
(2)![pH = -log [H_{3}O^{+}] = -log (0.00113) = 2.95](/tpl/images/0549/3755/8dc10.png)
![K_{b} = \frac{[C_{3}H_{6}O_{2}][OH^{-}]}{[C_{3}H_{5}O_{2}^{-}]}](/tpl/images/0549/3755/eac92.png)
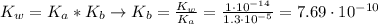
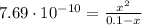 (3)
(3)![pOH = -log[OH^{-}] = -log(8.77\cdot 10^{-6}) = 5.06 \rightarrow pH = 14 - pOH = 8.94](/tpl/images/0549/3755/13708.png)
![K_{w} = [H^{+}][OH^{-}] \rightarrow 1\cdot 10^{-14} = [H^{+}][OH^{-}]](/tpl/images/0549/3755/f80a5.png)
![1\cdot 10^{-14} = [H^{+}]^{2} \rightarrow [H^{+}] = \sqrt{1\cdot 10^{-14}} = 1 \cdot 10^{-7}](/tpl/images/0549/3755/97d56.png)
![pH = -log [H^{+}] = -log (1 \cdot 10^{-7}) = 7.00](/tpl/images/0549/3755/acfd0.png)
![pH = pKa + log(\frac{[C_{3}H_{5}O_{2}^{-}]}{[C_{3}H_{6}O_{2}]}) = -log(1.3 \cdot 10^{-5}) + log(\frac{0.1}{0.1}) = 4.89](/tpl/images/0549/3755/7ff66.png)



