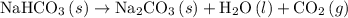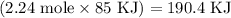Chemistry, 16.03.2020 21:15 msjsnell29
Given the following information: aHCO3(s)+85 kJ Na2CO3(s)+H2O(1) CO2(g) Calculate the amount of heat (ink) required to decompose 2.24 mol NaHCO3(s). You must show your work to receive credit .

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 01:00, carson9373
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1


Chemistry, 23.06.2019 15:00, Fangflora3
What do we call the rows on the periodic table? a. periodb. familyc. groupd. metals
Answers: 1

Chemistry, 23.06.2019 18:00, rio1228p7c7vj
If cos x =sin(20 + x) and 0 < x < 90, the value of x is > 3 , 35 , 350answer is 35, free 50 points
Answers: 1
You know the right answer?
Given the following information: aHCO3(s)+85 kJ Na2CO3(s)+H2O(1) CO2(g) Calculate the amount of heat...
Questions in other subjects:


Biology, 25.07.2019 00:30


Biology, 25.07.2019 00:30

History, 25.07.2019 00:30

Physics, 25.07.2019 00:30




Biology, 25.07.2019 00:30

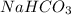 is shown below
is shown below