Chemistry, 16.03.2020 20:30 Tyrant4life
Many portable gas heaters and grills use propane, C3H8(g). Using enthalpies of formation, calculate the quantity of heat produced when 13.0 g of propane is completely combusted in air under standard conditions. Assume that liquid water is forming. Express the heat in kilojoules to three significant digits.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 04:50, psychocatgirl1
Write the overall equation for the reaction for lithium battery
Answers: 2

You know the right answer?
Many portable gas heaters and grills use propane, C3H8(g). Using enthalpies of formation, calculate...
Questions in other subjects:

Computers and Technology, 12.11.2020 17:30


Health, 12.11.2020 17:30

Chemistry, 12.11.2020 17:30

Mathematics, 12.11.2020 17:30

Mathematics, 12.11.2020 17:30



Social Studies, 12.11.2020 17:30

![\Delta H^o_{rxn}=\sum [n\times \Delta H_f_{(product)}]-\sum [n\times \Delta H_f_{(reactant)}]](/tpl/images/0549/0754/e893d.png)
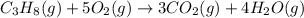
![\Delta H_{rxn}=[(3\times \Delta H_f_{(CO_2(g))})+(4\times \Delta H_f_{(H_2O(g))})]-[(1\times \Delta H_f_{(C_3H_8(g))})+(5\times \Delta H_f_{(O_2(g))})]](/tpl/images/0549/0754/b4bd0.png)
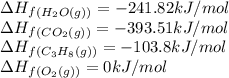
![\Delta H_{rxn}=[(3\times (-393.51))+(4\times (-241.82))]-[(1\times (-103.8))+(3\times (0))]\\\\\Delta H_{rxn}=-2044.01kJ/mol](/tpl/images/0549/0754/08c86.png)
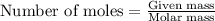
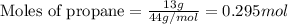
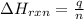
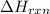 = enthalpy change of the reaction = -2044.01 kJ/mol
= enthalpy change of the reaction = -2044.01 kJ/mol