
Chemistry, 16.03.2020 20:09 ginareyes0423
Calculate ΔHrxnΔHrxn for the following reaction: 3C(s)+4H2(g)→C3H8(l)3C(s)+4H2(g)→C3 H8(l) Use the following reactions and given ΔHΔH values: C3H8(l)+5O2(g)→3CO2(g)+4H2O(g),ΔHC( s)+O2(g)→CO2(g),ΔH2H2(g)+O2(g)→2H2O (g),ΔH===−2026.6kJ−393.5kJ−483.5kJ

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 23:40, tilievaughn14
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1

You know the right answer?
Calculate ΔHrxnΔHrxn for the following reaction: 3C(s)+4H2(g)→C3H8(l)3C(s)+4H2(g)→C3 H8(l) Use the f...
Questions in other subjects:




Chemistry, 17.11.2019 00:31




Geography, 17.11.2019 00:31


Mathematics, 17.11.2019 00:31

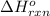 for the reaction is -120.9 kJ.
for the reaction is -120.9 kJ.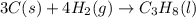
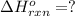
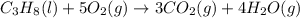
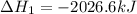
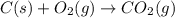
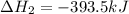 ( × 3)
( × 3)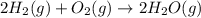
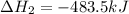 ( × 2)
( × 2)![\Delta H^o_{rxn}=[1\times (-\Delta H_1)]+[3\times \Delta H_2]+[2\times \Delta H_3]](/tpl/images/0548/9833/b4dbe.png)
![\Delta H^o_{rxn}=[(1\times -(-2026.6))+(3\times (-393.5))+(2\times (-483.5))]=-120.9kJ](/tpl/images/0548/9833/4ea2a.png)


