
Chemistry, 16.03.2020 18:59 nicollexo21
You are instructed to create 900. mL of a 0.29 M phosphate buffer with a pH of 7.8. You have phosphoric acid and the sodium salts NaH2PO4, Na2HPO4, and Na3PO4 available. (Enter all numerical answers to three significant figures.)
H3PO4(s) + H2O(l) <> H3O+(aq) + H2PO4−(aq) Ka1 = 6.9 ✕ 10−3
H2PO4−(aq) + H2O(l) <> H3O+(aq) + HPO42−(aq) Ka2 = 6.2 ✕ 10−8
HPO42−(aq) + H2O(l) <> H3O+(aq) + PO43−(aq) Ka3 = 4.8 ✕ 10−13
Which of the available chemicals will you use for the acid component of your buffer?
A. H3PO4
B. NaH2PO4
C. Na2HPO4
D. Na3PO4
Which of the available chemicals will you use for the base component of your buffer?
A. H3PO4B. NaH2PO4C. Na2HPO4D. Na3PO4

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, TMeansStupidity
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1


Chemistry, 22.06.2019 10:30, Wookas8355
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
You are instructed to create 900. mL of a 0.29 M phosphate buffer with a pH of 7.8. You have phospho...
Questions in other subjects:

Mathematics, 30.07.2019 15:00





History, 30.07.2019 15:00




![pKa_1=-Log[6.9x10^-^3]=2.16](/tpl/images/0548/8384/6a482.png)
![pKa_2=-Log[6.2x10^-^8]=7.20](/tpl/images/0548/8384/8337b.png)
![pKa_3=-Log[4.8x10^-^13]=12.31](/tpl/images/0548/8384/78632.png)
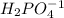 is the acid and
is the acid and  is the base. Therefore the answer for the first question is B and the answer for the second question is C.
is the base. Therefore the answer for the first question is B and the answer for the second question is C.


