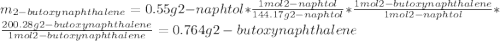Chemistry, 16.03.2020 18:34 daijafoster0
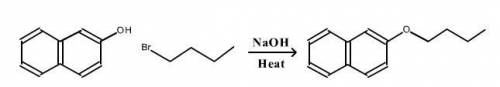
A reaction was performed in which 0.55 g of 2-naphthol was reacted with a slight excess of 1-bromobutane to make 0.32 g of 2-butoxynaphthalene. Calculate the theoretical yield and percent yield for this reaction.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 22:30, safiyabrowne7594
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1
You know the right answer?
A reaction was performed in which 0.55 g of 2-naphthol was reacted with a slight excess of 1-bromobu...
Questions in other subjects:

Mathematics, 25.09.2021 23:40

Mathematics, 25.09.2021 23:40





Mathematics, 25.09.2021 23:40


Biology, 25.09.2021 23:40

Social Studies, 25.09.2021 23:40