

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, mauifrifer3986
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 01:30, kayleg907436
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3
You know the right answer?
An unknown substance has a melting point of 1064°C, is insoluble in water, conducts electricity as a...
Questions in other subjects:



Physics, 27.06.2020 20:01


Physics, 27.06.2020 20:01

Mathematics, 27.06.2020 20:01




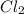 is a gas and it is made up of a non-metal. And, non-metals are poor conductors of heat and electricity therefore,
is a gas and it is made up of a non-metal. And, non-metals are poor conductors of heat and electricity therefore, 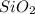 is a non-metallic compound therefore, it is unable to conduct electricity.
is a non-metallic compound therefore, it is unable to conduct electricity.  and it is insoluble in water and hard in nature. Also, it is able to conduct electricity in solid state.
and it is insoluble in water and hard in nature. Also, it is able to conduct electricity in solid state.

