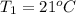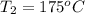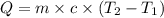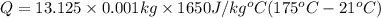Chemistry, 16.03.2020 18:16 deidaralove90
How much energy must be added to a bowl of 125popcorn kernels in order for them to reach a popping temperature of 175°C? Assume that their initial temperature is 21°C, that the specific heat capacity of popcorn is 1650 J/kg•°C, and that each kernel has a mass of 0.105 g.

Answers: 3


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 15:30, alaf05160
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks. energy was destroyed inside the blocks. energy was absorbed into the blocks from outside the system. energy was transferred from the warmer block to the cooler block.
Answers: 2
You know the right answer?
How much energy must be added to a bowl of 125popcorn kernels in order for them to reach a popping t...
Questions in other subjects:

Chemistry, 28.10.2020 18:30


Mathematics, 28.10.2020 18:30


Mathematics, 28.10.2020 18:30

Health, 28.10.2020 18:30


History, 28.10.2020 18:30

Mathematics, 28.10.2020 18:30