
Chemistry, 16.03.2020 17:57 graciearany
Given the following chemical reaction, calculate the number of moles of sodium sulfate produced when 3.25 moles of sodium chloride reacts with excess sulfuric acid according to the following balanced reaction; 2 NaCl(aq) + 1 H2SO4(aq) ---> 1 Na2SO4(aq) + 2 HCl(aq)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 22:30, jkjjoijjm5928
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 22.06.2019 23:40, tilievaughn14
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1
You know the right answer?
Given the following chemical reaction, calculate the number of moles of sodium sulfate produced when...
Questions in other subjects:

Chemistry, 11.11.2020 05:00

Biology, 11.11.2020 05:00


Mathematics, 11.11.2020 05:00

Health, 11.11.2020 05:00


Chemistry, 11.11.2020 05:00

Mathematics, 11.11.2020 05:00


Arts, 11.11.2020 05:00

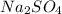 produced is, 1.62 moles
produced is, 1.62 moles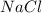 = 3.25 mol
= 3.25 mol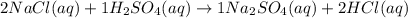
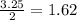 mole of
mole of 

