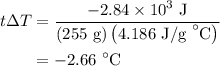Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, rileyeddins1010
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 23:30, hcllxxhhlpcj
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 04:20, tyrickdavis1
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1
You know the right answer?
An 8.5g ice cube is placed into 255g of water. calculate the temperature change in the water upon th...
Questions in other subjects:

Mathematics, 13.10.2020 05:01


Mathematics, 13.10.2020 05:01

Social Studies, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01


World Languages, 13.10.2020 05:01

 .
.
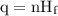 …… (1)
…… (1)
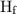 is latent heat of fusion.
is latent heat of fusion.
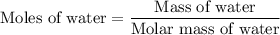 …… (2)
…… (2)
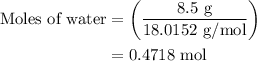
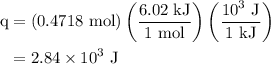
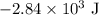 .
.
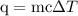 …… (3)
…… (3)
 is change in temperature.
is change in temperature.
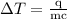 …… (4)
…… (4)
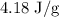 for c in equation (4).
for c in equation (4).