Chemistry, 16.03.2020 17:33 20emmanuelg1030
Write the precipitation reaction for calcium chloride in aqueous solution: Use the pull-down menus to specify the state of each reactant and product. CaCl2 + Is calcium chloride considered soluble or not soluble ? ... ... A. Soluble ... B. Not soluble

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 16:00, graciewyatt6833
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
Write the precipitation reaction for calcium chloride in aqueous solution: Use the pull-down menus t...
Questions in other subjects:




History, 10.12.2020 20:00

Mathematics, 10.12.2020 20:00

Mathematics, 10.12.2020 20:00


Biology, 10.12.2020 20:00


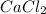 is an ionic compound as it's atoms contain a metal and a non-metal.
is an ionic compound as it's atoms contain a metal and a non-metal.