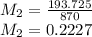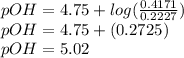Chemistry, 16.03.2020 16:59 dlewis9632
A buffer is made by combining 5.55 g of NH3(Kb= 1.8 X 10-5)with 4.78 g of HCl and diluting to a volume of 750.0 mL. a. What is the pH?b. What is the pH after adding 60.0 mL of 2.00 M HBr(aq)?c. What is the pH after adding 120.0 mL of 2.00 M HBr(aq)?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, AIhunter2884
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 06:30, noathequeen
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

You know the right answer?
A buffer is made by combining 5.55 g of NH3(Kb= 1.8 X 10-5)with 4.78 g of HCl and diluting to a volu...
Questions in other subjects:

Mathematics, 04.09.2020 20:01

Mathematics, 04.09.2020 20:01





Mathematics, 04.09.2020 20:01


English, 04.09.2020 20:01

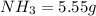
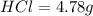
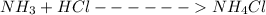
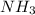 = 17 g/mol
= 17 g/mol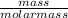
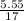
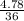
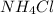
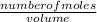
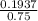
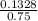
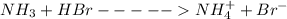
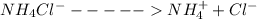
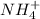 is present in both of the above reactions; then this a buffer solution which is demonstrated by using Henderson Hasslelbach equation.
is present in both of the above reactions; then this a buffer solution which is demonstrated by using Henderson Hasslelbach equation.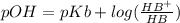
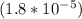
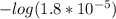
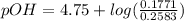
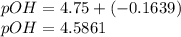
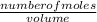
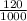
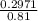
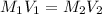 ; we have :
; we have :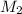 × 810
× 810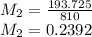
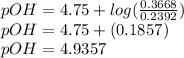
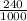
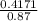
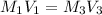 ; we have :
; we have :