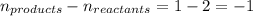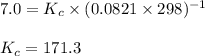The equilibrium between NO2 and N2O4 can be described by the following equation:
2NO2(g...

Chemistry, 16.03.2020 16:11 maddie53116
The equilibrium between NO2 and N2O4 can be described by the following equation:
2NO2(g) ⇌ N2O4(g) Kp = 7.0
If a sealed flask contains 1.5 atm of NO2 and 14.2 atm of N2O4. Calculate the value of Kc for the reaction,

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:30, artemiscrock77041
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 21.06.2019 21:30, gatorr2010
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 07:30, SchoolFirst9811
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н, о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2
You know the right answer?
Questions in other subjects:

Mathematics, 17.11.2020 04:50


Mathematics, 17.11.2020 04:50

History, 17.11.2020 04:50


History, 17.11.2020 04:50




History, 17.11.2020 04:50

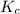 for given reaction is 171.3
for given reaction is 171.3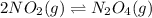
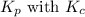 is given by the formula:
is given by the formula: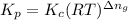
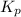 = equilibrium constant in terms of partial pressure = 7.0
= equilibrium constant in terms of partial pressure = 7.0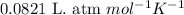
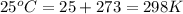
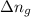 = change in number of moles of gas particles =
= change in number of moles of gas particles =