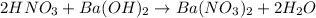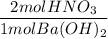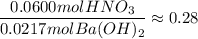Chemistry, 16.03.2020 06:22 kameronstebbins
If 10.0 mL of a .600 M of HNO3 reacts with 31.0 mL of .700M Ba(OH)2 solution, what is the molarity of Ba(OH)2 after the reaction is complete

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 23.06.2019 00:30, vane6176
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3

Chemistry, 23.06.2019 01:30, nikonee
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1

Chemistry, 23.06.2019 22:00, limitbreak7897
Define a function compute_gas_volume that returns the volume of a gas given parameters pressure, temperature, and moles. use the gas equation pv = nrt, where p is pressure in pascals, v is volume in cubic meters, n is number of moles, r is the gas constant 8.3144621 ( j / (mol* and t is temperature in kelvin.
Answers: 2
You know the right answer?
If 10.0 mL of a .600 M of HNO3 reacts with 31.0 mL of .700M Ba(OH)2 solution, what is the molarity o...
Questions in other subjects:

English, 23.04.2021 23:40


Mathematics, 23.04.2021 23:40

Mathematics, 23.04.2021 23:40

Mathematics, 23.04.2021 23:40


English, 23.04.2021 23:40