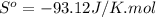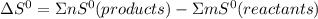Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 09:30, lisbet123085
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 15:30, abdullaketbi71
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
Calculate the standard entropy change for the following reaction cu(s) + 1/2 O2(g) —> cuo(s)...
Questions in other subjects:


Mathematics, 08.11.2020 01:00

Mathematics, 08.11.2020 01:00


Computers and Technology, 08.11.2020 01:00

English, 08.11.2020 01:00


Mathematics, 08.11.2020 01:00


Mathematics, 08.11.2020 01:00

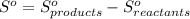
![S^o = [42.59] - [33.17 + \frac{205.07}{2}] J/K.mol](/tpl/images/0548/0970/feb44.png)
![S^o = [42.59] - [33.17 + 102.535] J/K.mol](/tpl/images/0548/0970/702bd.png)
![S^o = [42.59] - [135.705] J/K.mol](/tpl/images/0548/0970/7dc74.png)