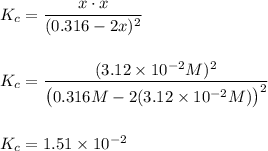A student ran the following reaction in the laboratory at 636 K:
2HI(g) -> H2(g) + I2...

A student ran the following reaction in the laboratory at 636 K:
2HI(g) -> H2(g) + I2(g)
When she introduced 0.316 moles of HI(g) into a 1.00 liter container, she found the equilibrium concentration of I2(g) to be 3.12×10-2 M.
Calculate the equilibrium constant, Kc, she obtained for this reaction.
Kc =

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 16:00, ghadeeraljelawy
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2


Chemistry, 22.06.2019 21:30, leenzazou587
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 20.03.2020 21:30

History, 20.03.2020 21:30


Spanish, 20.03.2020 21:30

Mathematics, 20.03.2020 21:30

English, 20.03.2020 21:30


Mathematics, 20.03.2020 21:30


Mathematics, 20.03.2020 21:30