
Chemistry, 14.03.2020 06:55 rosemarybooker
The following reaction is spontaneous as written when the components are in their standard states:
3 Zn(s) +2 Cr3+(aq) →3 Zn2+(aq) +2 Cr(s)
If the [Zn2+] is 4 molL−1, determine the value of [Cr3+] below which the reaction will be spontaneous in the opposite direction.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2
You know the right answer?
The following reaction is spontaneous as written when the components are in their standard states:
Questions in other subjects:


Mathematics, 05.02.2021 01:20

Chemistry, 05.02.2021 01:20

Mathematics, 05.02.2021 01:20

Mathematics, 05.02.2021 01:20


Mathematics, 05.02.2021 01:20

Arts, 05.02.2021 01:20

History, 05.02.2021 01:20

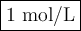

![\begin{array}{rcl}Q & = & \dfrac{\text{[Zn$^{2+}$]$^{3}$}}{\text{[Cr}^{3+}]^{2}}\\\\64.0 & = & \dfrac{4^{3}}{\text{[Cr}^{3+}]^{2}}\\\\\text{[Cr}^{3+}]^{2}& = & \dfrac{64}{64.0}\\\\& = & 1\\\text{[Cr}^{3+}] & = & \textbf{1 mol/L}\\\end{array}\\\text{[Cr$^{3+}$] must be less than $\large \boxed{\textbf{1 mol/L}}$ for the reaction to be spontaneous in the reverse}\\\text{direction.}](/tpl/images/0547/6685/5fcfe.png)


