
Chemistry, 13.03.2020 23:58 jwood287375
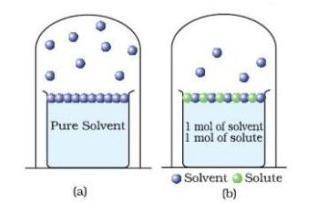
Use the model below to answer the question.
A. Student B says adding an ionic solute increases the boiling point more than adding a covalent solute.
B. Student D says adding a solute allows more water to leave (decreases the boiling point)
C. Student A says adding a solute allows less water to leave (increases the boiling point)
D. Student C says adding a solute has no effect on the boiling point

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 14:00, daniel1480
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 14:20, kekecantonxox121
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1
You know the right answer?
Use the model below to answer the question.
A. Student B says adding an ionic solute increase...
A. Student B says adding an ionic solute increase...
Questions in other subjects:

History, 05.05.2020 04:13

Mathematics, 05.05.2020 04:13

Mathematics, 05.05.2020 04:13

Mathematics, 05.05.2020 04:13


Advanced Placement (AP), 05.05.2020 04:13

Mathematics, 05.05.2020 04:13






