Chemistry, 13.03.2020 23:58 gwendallinesikes
Determine the value of Kc for the following reaction if the equilibrium concentrations are as follows: [N2]eq = 1.5 M, [H2]eq = 1.1 M, [NH3]eq = 0.47 M. N2(g) + 3 H2(g) 2 NH3(g)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 23.06.2019 08:00, mshields1994
Amechanical wave that transports a lot of energy will have a
Answers: 2
You know the right answer?
Determine the value of Kc for the following reaction if the equilibrium concentrations are as follow...
Questions in other subjects:


Biology, 12.04.2020 21:34




Mathematics, 12.04.2020 21:34

Mathematics, 12.04.2020 21:34

Mathematics, 12.04.2020 21:34

Social Studies, 12.04.2020 21:34

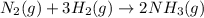
![K_c=\frac{[NH_3]^2}{[N_2][H_2]^3}](/tpl/images/0547/2110/c3aa0.png)