
Oxygen gas reacts with powdered aluminum according to the following reaction:
4Al(s)+3O2(g)→2Al2O3(s)
What volume of O2 gas, measured at 787 mmHg and 21 ∘C, is required to completely react with 55.0 g of Al?
Express the volume in liters to three significant figures.

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 14:40, neonbluefaith
Which statement best describes the function of enzymes?
Answers: 1
You know the right answer?
Oxygen gas reacts with powdered aluminum according to the following reaction:
4Al(s)+3O2...
4Al(s)+3O2...
Questions in other subjects:

Social Studies, 22.10.2020 14:01

Mathematics, 22.10.2020 14:01








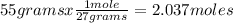
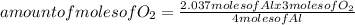
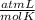 T= 21 C=294 K
T= 21 C=294 K


