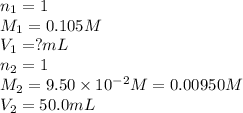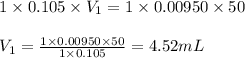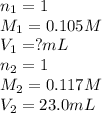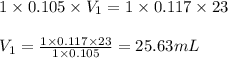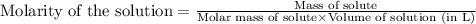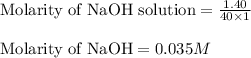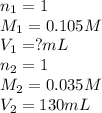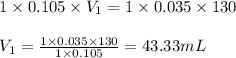Chemistry, 13.03.2020 22:21 WritingStar1313
How many milliliters of 0.105 M HCl are needed to titrate each of the following solutions to the equivalence point?
a. 50.0 mL of 9.5010?2 M NaOH
b. 23.0 mL of 0.117 M NH3
c. 130 mL of a solution that contains 1.40 g of NaOH per liter

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, johnnydenali67
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 16:50, TheOriginal2x
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 19:30, jessixa897192
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
How many milliliters of 0.105 M HCl are needed to titrate each of the following solutions to the equ...
Questions in other subjects:










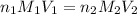
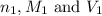 are the n-factor, molarity and volume of acid
are the n-factor, molarity and volume of acid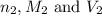 are the n-factor, molarity and volume of base
are the n-factor, molarity and volume of base