
Chemistry, 13.03.2020 20:03 LilFreaky666
Consider these reactions, where M represents a generic metal.
2 M (s) + 6 HCl (aq) ⟶ 2 MCl₃ (aq) + 3 H₂ (g); ΔH₁ = − 878.0 k J
HCl (g) ⟶ HCl (aq); ΔH₂ = − 74.8 k J
H₂ (g) + Cl₂ (g) ⟶ 2 HCl (g); ΔH₃ = − 1845.0 k J
MCl₃ (s) ⟶ MCl₃ ( aq ); ΔH₄ = − 497.0 k J
Use the given information to determine the enthalpy of the reaction
2 M (s) + 3 Cl₂ (g) ⟶ 2 MCl₃ (s).

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, 20alondra04
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1


Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
Consider these reactions, where M represents a generic metal.
2 M (s) + 6 HCl (aq) ⟶ 2 MCl₃ (...
2 M (s) + 6 HCl (aq) ⟶ 2 MCl₃ (...
Questions in other subjects:




History, 26.06.2019 00:40



Mathematics, 26.06.2019 00:40



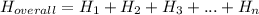 .
.

