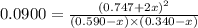Chemistry, 13.03.2020 19:56 donttrip10
A mixture of 0.590 M H 2 O , 0.340 M Cl 2 O , and 0.747 M HClO are enclosed in a vessel at 25 ° C . H 2 O ( g ) + Cl 2 O ( g ) − ⇀ ↽ − 2 HOCl ( g ) K c = 0.0900 at 25 ° C Calculate the equilibrium concentrations of each gas at 25 ° C .

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 02:30, erikacastro259
what is your question? collegechemistry 5+3 pts in november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3

Chemistry, 23.06.2019 04:10, angelina6836
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2

Chemistry, 23.06.2019 15:30, Ponypepper5699
What is the predicted change in the boiling point of water when 1.50 g of barium chloride is dissolved in 1.50 kg of water?
Answers: 3
You know the right answer?
A mixture of 0.590 M H 2 O , 0.340 M Cl 2 O , and 0.747 M HClO are enclosed in a vessel at 25 ° C ....
Questions in other subjects:

Mathematics, 10.11.2020 04:40

Mathematics, 10.11.2020 04:40



Mathematics, 10.11.2020 04:40

Mathematics, 10.11.2020 04:40

Biology, 10.11.2020 04:40



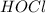 ,
, 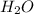 and
and 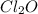 at equilibrium is, 0.215 M, 0.856 M and 0.606 M respectively.
at equilibrium is, 0.215 M, 0.856 M and 0.606 M respectively.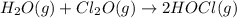
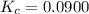
![K_c=\frac{[HOCl]^2}{[H_2O][Cl_2O]}](/tpl/images/0546/8405/da783.png)