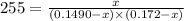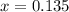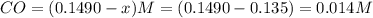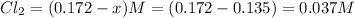CO (g) + Cl2 (g) ⇌ COCl2 (g)

Chemistry, 13.03.2020 19:17 owenbarrows
For the following reaction, Kc = 255 at 1000 K.
CO (g) + Cl2 (g) ⇌ COCl2 (g)
A reaction mixture initially contains a CO concentration of 0.1490 M and a Cl2 concentration of 0.172 M at 1000 K.
Part A
What is the equilibrium concentration of CO at 1000 K?
Express your answer in molarity to three significant figures.
Part B
What is the equilibrium concentration of Cl2 at 1000 KK?
Express your answer in molarity to three significant figures.
Part C
What is the equilibrium concentration of COCl2 at 1000 KK?
Express your answer in molarity to three significant figures.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, nothingworksoutforme
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1


Chemistry, 22.06.2019 07:30, deidaraXneji
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 17:20, phanuel642
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
You know the right answer?
For the following reaction, Kc = 255 at 1000 K.
CO (g) + Cl2 (g) ⇌ COCl2 (g)
CO (g) + Cl2 (g) ⇌ COCl2 (g)
Questions in other subjects:


Mathematics, 26.01.2021 03:50





Mathematics, 26.01.2021 03:50

Advanced Placement (AP), 26.01.2021 04:00

Chemistry, 26.01.2021 04:00

Mathematics, 26.01.2021 04:00

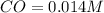
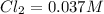

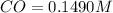
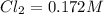
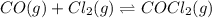
![K_c=\frac{[COCl_2]}{[Cl_2]\times [CO]}](/tpl/images/0546/7266/b0e05.png)