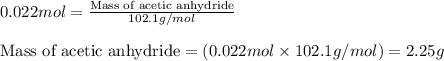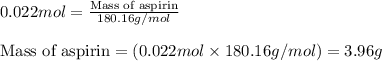Chemistry, 13.03.2020 18:34 chloeann5397
Aspirin is prepared by reaction of salicylic acid (C7H6O3) with acetic anhydride (C4H6O3)according to the following equation:
C7H6O3Salicylicacid+C4H6O3Aceticanh ydride→C9H8O4Aspirin+CH3COOHAcetica cid
a. How many grams of acetic anhydride are needed to react with 3.07 g of salicylic acid?
b. How many grams of aspirin will result?
c. How many grams of acetic acid are formed as a by-product?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, Jazmineboo7709
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2


Chemistry, 23.06.2019 15:30, tymiahill7244
Iv the concentration of ionic substances is important for the heart to beat. your heart responds to electrical impulses that travel through heart cells that are made up mostly of water. which properties of ionic compounds are important to support this function? solubility in water conductivity crystalline melting point الم" الا به done رلرلرللللہ و و او 8
Answers: 1
You know the right answer?
Aspirin is prepared by reaction of salicylic acid (C7H6O3) with acetic anhydride (C4H6O3)according t...
Questions in other subjects:

Mathematics, 16.05.2021 21:00

Social Studies, 16.05.2021 21:00


Physics, 16.05.2021 21:00

Mathematics, 16.05.2021 21:00


Physics, 16.05.2021 21:00

English, 16.05.2021 21:00


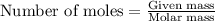 .....(1)
.....(1)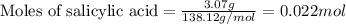
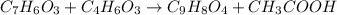
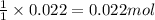 of acetic anhydride
of acetic anhydride