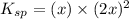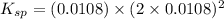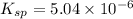Chemistry, 13.03.2020 18:35 ilovecatsomuchlolol
Excess Ca(OH)2 is shaken with water to produce a saturated solution. The solution is filtered, and a 50.00 mL sample titrated with HCl requires 11.15 mL of 0.0973 M HCl to reach the end point. calculate ksp for Ca(OH)2?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, caitybugking
Type the correct answer in the box. spell all words correctly. what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 08:30, itzhari101
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1


Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
Excess Ca(OH)2 is shaken with water to produce a saturated solution. The solution is filtered, and a...
Questions in other subjects:

Mathematics, 14.12.2021 02:20



Mathematics, 14.12.2021 02:20





Social Studies, 14.12.2021 02:20

Mathematics, 14.12.2021 02:20

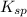 is
is 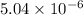
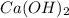 , we use the equation given by neutralization reaction:
, we use the equation given by neutralization reaction: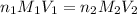
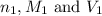 are the n-factor, molarity and volume of
are the n-factor, molarity and volume of 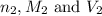 are the n-factor, molarity and volume of HCl.
are the n-factor, molarity and volume of HCl.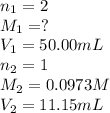
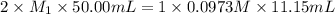
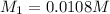
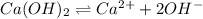
![K_{sp}=[Ca^{2+}][OH^{-}]^2](/tpl/images/0546/6867/4ff7a.png)