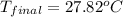Chemistry, 13.03.2020 05:01 afolmar2006
A 26.4 g sample of aluminum at 100.6 °C is added to 100.4 g of water at 22.0 °C in a constant pressure calorimeter. What is the final temperature of the water in °C? The specific heat capacity of aluminum is 0.903 J/g°C .

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:50, ddmoorehouseov75lc
If a substance is not at its melting or boiling point, as the heat content of a sample of matter increases, its temperature increases the number of intermolecular bonds decreases the space between particles increases the particles move faster
Answers: 2

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 21:00, taylorlanehart
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
A 26.4 g sample of aluminum at 100.6 °C is added to 100.4 g of water at 22.0 °C in a constant pressu...
Questions in other subjects:


History, 18.10.2021 07:30


Mathematics, 18.10.2021 07:30



Mathematics, 18.10.2021 07:30

Chemistry, 18.10.2021 07:30


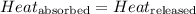
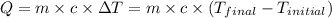
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0546/2501/09236.png) ......(1)
......(1)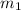 = mass of aluminium = 26.4 g
= mass of aluminium = 26.4 g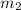 = mass of water = 100.4 g
= mass of water = 100.4 g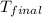 = final temperature = ?°C
= final temperature = ?°C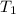 = initial temperature of aluminium = 100.6°C
= initial temperature of aluminium = 100.6°C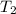 = initial temperature of water = 22.0°C
= initial temperature of water = 22.0°C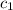 = specific heat of aluminium = 0.903 J/g°C
= specific heat of aluminium = 0.903 J/g°C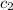 = specific heat of water= 4.186 J/g°C
= specific heat of water= 4.186 J/g°C![26.4\times 0.903\times (T_{final}-100.6)=-[100.6\times 4.186\times (T_{final}-23.7)]](/tpl/images/0546/2501/b8661.png)