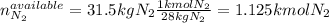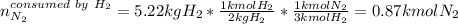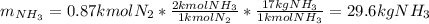Chemistry, 13.03.2020 02:27 ddddre3909
Ammonia can also be synthesized by the reaction: 3H2(g)+N2(g)→2NH3(g) What is the theoretical yield of ammonia, in kg, that we can synthesize from 5.22 kg of H2 and 31.5 kg of N2? Express the mass in kilograms to three significant figures.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 14:50, ladybugperez05
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
Ammonia can also be synthesized by the reaction: 3H2(g)+N2(g)→2NH3(g) What is the theoretical yield...
Questions in other subjects:

Mathematics, 14.07.2020 23:01





Mathematics, 14.07.2020 23:01

Mathematics, 14.07.2020 23:01

Mathematics, 14.07.2020 23:01

Mathematics, 14.07.2020 23:01