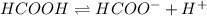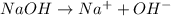Chemistry, 13.03.2020 01:16 kimbely7704
Which statement is correct?Strong bases are good electrolytes because they completely ionize in water. Strong bases are poor electrolytes because they partially ionize in water. Strong bases are poor electrolytes because they completely ionize in water. Strong bases are good electrolytes because they partially ionize in water.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, AbhiramAkella
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 18:30, losalobos46
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
Which statement is correct?Strong bases are good electrolytes because they completely ionize in wate...
Questions in other subjects:



Mathematics, 30.03.2021 17:40

Mathematics, 30.03.2021 17:40

Mathematics, 30.03.2021 17:40


History, 30.03.2021 17:40

History, 30.03.2021 17:40


English, 30.03.2021 17:40