Chemistry, 25.08.2019 17:50 fardinhaque6113
Suppose you have a calorimeter that contains 100.0 grams of water at an initial temperature of 25*c. a salt (2.19 g, 0.020 moles) is dissolved in the water, and the final temperature is 29*c. calculate the standard heat of solution (on a per mole basis). was the dissolution exothermic or endothermic?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, ciarakelly636owuiup
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 22.06.2019 05:20, anggar20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
You know the right answer?
Suppose you have a calorimeter that contains 100.0 grams of water at an initial temperature of 25*c....
Questions in other subjects:

Spanish, 06.11.2020 03:50






English, 06.11.2020 03:50

English, 06.11.2020 03:50

Advanced Placement (AP), 06.11.2020 03:50

Mathematics, 06.11.2020 03:50

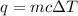
 = change in temperature =
= change in temperature = 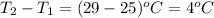
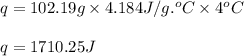
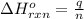
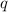 = amount of heat absorbed = 1710.25 J
= amount of heat absorbed = 1710.25 J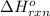 = standard enthalpy change of the reaction
= standard enthalpy change of the reaction