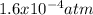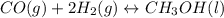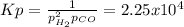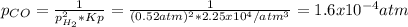Chemistry, 12.03.2020 22:09 demetriascott20
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particular temperature. Determine the equilibrium pressure of CO. CO(g) + 2 H2(g) CH3OH(l) Kp = 2.25 × 104 P(H2)eq = 0.52 atm

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, darrriannn7241
What is the correct lewis structure for chloroform chcl3
Answers: 1

Chemistry, 22.06.2019 07:20, JKINGblackstar3502
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 14:00, luisaareli6298
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1
You know the right answer?
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particula...
Questions in other subjects:

Mathematics, 30.06.2019 02:20

Chemistry, 30.06.2019 02:20



Mathematics, 30.06.2019 02:20

French, 30.06.2019 02:20