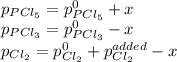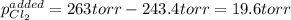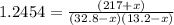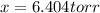Chemistry, 12.03.2020 21:01 jsmith4184
An equilibrium mixture of PCl 5 ( g ) , PCl 3 ( g ) , and Cl 2 ( g ) has partial pressures of 217.0 Torr, 13.2 Torr, and 13.2 Torr, respectively. A quantity of Cl 2 ( g ) is injected into the mixture, and the total pressure jumps to 263.0 Torr at the moment of mixing. The system then re-equilibrates. The chemical equation for this reaction is PCl 3 ( g ) + Cl 2 ( g ) − ⇀ ↽ − PCl 5 ( g ) Calculate the new partial pressures, P , after equilibrium is reestablished.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, taylorpayne525p8qxky
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3


Chemistry, 23.06.2019 02:00, xoxoadara13ox07ck
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
An equilibrium mixture of PCl 5 ( g ) , PCl 3 ( g ) , and Cl 2 ( g ) has partial pressures of 217.0...
Questions in other subjects:


History, 29.03.2020 03:00

Mathematics, 29.03.2020 03:00

Mathematics, 29.03.2020 03:00

Social Studies, 29.03.2020 03:00

Mathematics, 29.03.2020 03:00



Mathematics, 29.03.2020 03:00

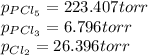
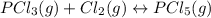
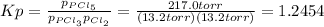
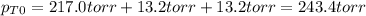
 owing to the chlorine's addition, turn out:
owing to the chlorine's addition, turn out: