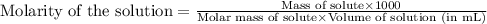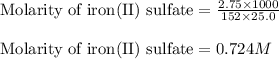Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 07:20, JKINGblackstar3502
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1
You know the right answer?
An aqueous solution of iron(II) sulfate (FeSO4) is prepared by dissolving 2.75 g in sufficient deion...
Questions in other subjects:

Biology, 08.07.2019 08:30

Health, 08.07.2019 08:30



Computers and Technology, 08.07.2019 08:30


Mathematics, 08.07.2019 08:30

Chemistry, 08.07.2019 08:30

Mathematics, 08.07.2019 08:30