Chemistry, 12.03.2020 17:25 aubreymoore4553
What is the value of the van't Hoff factor for KCl if a 1.00m aqueous solution shows a vapor pressure depression of 0.734 mmHg at 298 ∘C? (The vapor pressure of water at 298 K is 23.76 mmHg.)

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 21:30, sullivanjakob
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 23.06.2019 01:30, nikonee
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
What is the value of the van't Hoff factor for KCl if a 1.00m aqueous solution shows a vapor pressur...
Questions in other subjects:

Biology, 14.01.2021 21:30

Mathematics, 14.01.2021 21:30

History, 14.01.2021 21:30


English, 14.01.2021 21:30

Biology, 14.01.2021 21:30




Biology, 14.01.2021 21:30

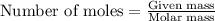
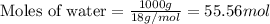
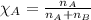
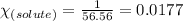
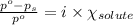
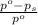 = relative lowering in vapor pressure = 0.734 mmHg
= relative lowering in vapor pressure = 0.734 mmHg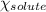 = mole fraction of solute = 0.0177
= mole fraction of solute = 0.0177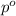 = vapor pressure of pure water = 23.76 torr
= vapor pressure of pure water = 23.76 torr