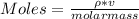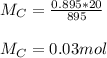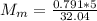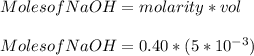You added 5.00 mL of 0.40 M NaOH in methanol to 20.00 mL of cooking oil. Calculate the number of moles of vegetable oil, methanol, and NaOH that are initially present in the sample. Assume the density of vegetable oil is 0.895 g/mL and the molar mass is 895 g/mol. Look up the density and molar mass of any other compounds as needed.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, BornAdopted21
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2


Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 14:50, wcraig1998
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1
You know the right answer?
You added 5.00 mL of 0.40 M NaOH in methanol to 20.00 mL of cooking oil. Calculate the number of mol...
Questions in other subjects:

Mathematics, 22.02.2021 18:20


Mathematics, 22.02.2021 18:20

Mathematics, 22.02.2021 18:20



Mathematics, 22.02.2021 18:20

Mathematics, 22.02.2021 18:20

Mathematics, 22.02.2021 18:20

Mathematics, 22.02.2021 18:20