
Chemistry, 12.03.2020 05:41 Rachelmontes1
A 25-kg iron block initially at 350 oC is quenched in an insulated tank that contains 100 kg of water at 18 oC. Assuming the water that vaporizes during the process condenses back in the tank, determine the total entropy change during this process.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 03:00, duplessistoccara
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2

Chemistry, 23.06.2019 03:00, Cheyenne7327
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1

Chemistry, 23.06.2019 05:20, cjking2320
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2
You know the right answer?
A 25-kg iron block initially at 350 oC is quenched in an insulated tank that contains 100 kg of wate...
Questions in other subjects:


English, 20.08.2020 20:01


Mathematics, 20.08.2020 20:01

Mathematics, 20.08.2020 20:01

Mathematics, 20.08.2020 20:01

English, 20.08.2020 20:01



Chemistry, 20.08.2020 20:01

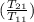 = 25 kg×0.444 J/g·°C㏑
= 25 kg×0.444 J/g·°C㏑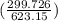 = -8124.296 J/K = -8.124 kJ/K
= -8124.296 J/K = -8.124 kJ/K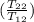 = 100 kg×4.186 J/g·°C㏑
= 100 kg×4.186 J/g·°C㏑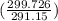 = 12152.01 J/K = 12.15201 kJ/K
= 12152.01 J/K = 12.15201 kJ/K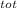 = ΔS₁ + ΔS₂ = -8.124 kJ/K + 12.15201 kJ/K = 4.03 kJ/K.
= ΔS₁ + ΔS₂ = -8.124 kJ/K + 12.15201 kJ/K = 4.03 kJ/K.

