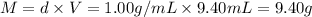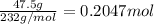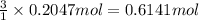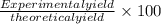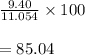Chemistry, 12.03.2020 05:32 kfcnkfnmnfk9513
What is the percent yield of a reaction in which 47.5 g tungsten (VI) oxide (WO3) reacts with excess hydrogen gas to produce metallic tungsten and 9.40 mL water (d = 1.00 g/mL)?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, ElizabethF
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 03:00, litttyyyu33411
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2


Chemistry, 22.06.2019 21:30, crystalbyrd79p8imrx
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
What is the percent yield of a reaction in which 47.5 g tungsten (VI) oxide (WO3) reacts with excess...
Questions in other subjects:


Mathematics, 12.06.2020 18:57

Mathematics, 12.06.2020 18:57

Social Studies, 12.06.2020 18:57

English, 12.06.2020 18:57





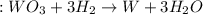
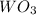 = 47.5g
= 47.5g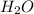 = 18 g/mole
= 18 g/mole