

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:10, RealStephani
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(s o4)2·7h2omgso4·7h2o
Answers: 1


You know the right answer?
A balloon contains 30.0 L of helium gas at 1.00 atm. What is the volume of the helium when the ballo...
Questions in other subjects:


Health, 02.10.2019 03:30

Chemistry, 02.10.2019 03:30






History, 02.10.2019 03:30

Social Studies, 02.10.2019 03:30

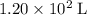 at a pressure of
at a pressure of 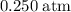 .
. 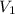 and
and 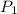 be the volume and pressure of the gas before the change.
be the volume and pressure of the gas before the change. 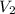 and
and 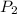 represent the volume after the change, then
represent the volume after the change, then 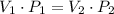 .
. 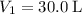 .
.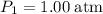 .
.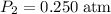 .
.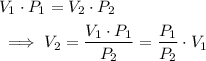 .
.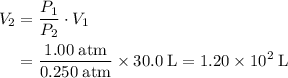 .
.


