

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1


Chemistry, 23.06.2019 22:00, DnsMonsteR7712
How many grams of water are in 19.2 moles of water? *#28 is the question
Answers: 1
You know the right answer?
It takes 12.45 milliliters of 0.50 M NAOH to back titrate a mixture containing 1.25 grams of baking...
Questions in other subjects:


Mathematics, 03.05.2021 21:50

History, 03.05.2021 21:50



Mathematics, 03.05.2021 21:50

Mathematics, 03.05.2021 21:50

Social Studies, 03.05.2021 21:50

History, 03.05.2021 21:50

 (as 1 L = 1000 ml)
(as 1 L = 1000 ml) (12.45 ml = 0.1245 L)
(12.45 ml = 0.1245 L)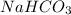 will be as follows.
will be as follows.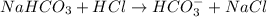
 . Hence, moles of
. Hence, moles of 
 .
. 


