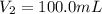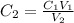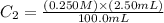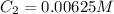Chemistry, 12.03.2020 03:01 toledanomariap43bxm
Determine the concentration of the following dye standard made by a student who pipettes out 2.50 mL of a 0.250 M stock solution and transfers it into a volumetric flask and dilutes the dye to a final volume of 100.0 mL with DI water.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, lindseyklewis1p56uvi
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1


Chemistry, 22.06.2019 08:00, juliannxkim
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

You know the right answer?
Determine the concentration of the following dye standard made by a student who pipettes out 2.50 mL...
Questions in other subjects:


Mathematics, 27.03.2020 20:35

Mathematics, 27.03.2020 20:35




Mathematics, 27.03.2020 20:35



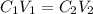
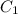 and
and 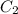 are initial and final concentration respectively
are initial and final concentration respectively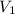 and
and 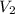 are initial and final volume respectively
are initial and final volume respectively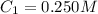 ,
, 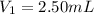 and
and