Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:30, bibiansolis
The table lists the lattice energies of some compounds. compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf. the lattice energy increases as the cations get larger, as shown by lif and licl. the lattice energy decreases as cations get smaller, as shown by nacl and naf. the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3
You know the right answer?
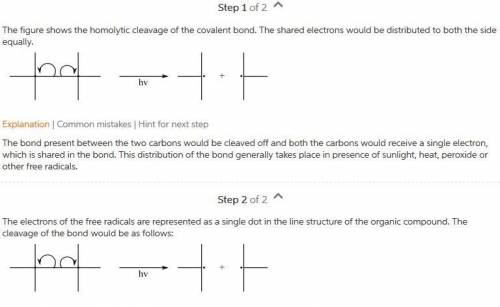
Draw the products of the transformation shown by the fishhook notation. Include any electrons....
Questions in other subjects:

Mathematics, 15.01.2021 20:20


Biology, 15.01.2021 20:20

Mathematics, 15.01.2021 20:20

Business, 15.01.2021 20:20

Advanced Placement (AP), 15.01.2021 20:20

Mathematics, 15.01.2021 20:20

English, 15.01.2021 20:20

Mathematics, 15.01.2021 20:20

Advanced Placement (AP), 15.01.2021 20:20