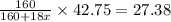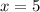Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 19:00, HaydenSturgis1
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 23.06.2019 01:20, michellectucker1982
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 02:20, theactualslash
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
You know the right answer?
A chemist is given a sample of the CuSO4 hydrate and asked to determine its the empirical formula. T...
Questions in other subjects:


History, 20.08.2019 12:10


Mathematics, 20.08.2019 12:10



Mathematics, 20.08.2019 12:10

Mathematics, 20.08.2019 12:10




 = 160 g/mol
= 160 g/mol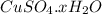 decomposes to give 160 g of anhydrous
decomposes to give 160 g of anhydrous  g of anhydrous
g of anhydrous